I predict a brave new world in which a significant proportion of the population will be receiving pharmacological interventions to optimise the ageing process. #BrainHealth #PreventiveNeurology
The following study is a good example of the revolution that genomics has brought to the practice of medicine.
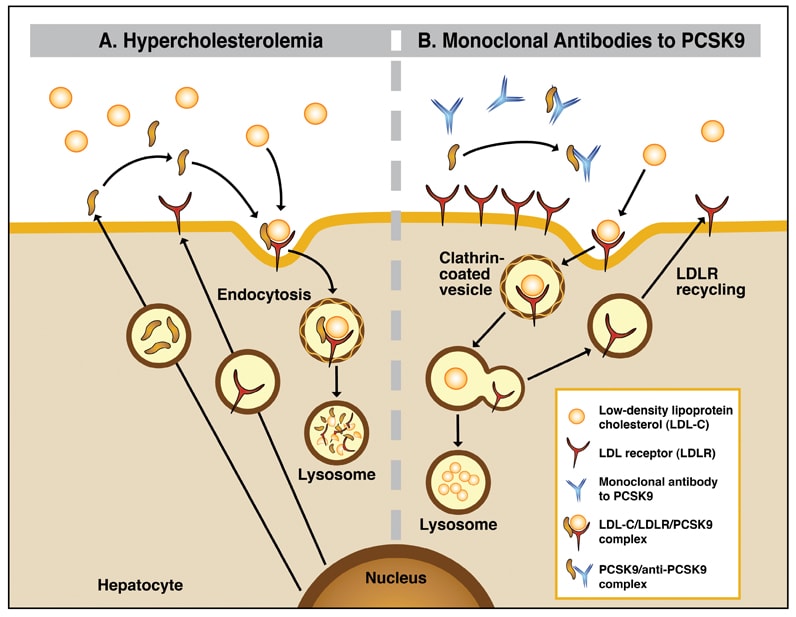
The discovery (2003) that gain-of-function mutations in the proprotein convertase subtilisin–kexin type 9 (PCSK9) gene causes autosomal dominant hypercholesterolaemia, and the identification (2005) of loss-of-function mutations in PCSK9 causing lower low-density lipoprotein (LDL) cholesterol levels, led to the PCSK9 pathway becoming a therapeutic target.
PCSK9 is a secreted serine protease that binds to the extracellular domain of the LDL receptor and targets the LDL receptor to the lysosomal compartment for degradation. PCSK9 prevents recycling of the LDL receptor to the cell surface, thereby attenuating LDL clearance. PCSK9 circulates in plasma, where it is closely associated with lipoprotein particles. Higher circulating PCSK9 levels predict lower catabolism of apolipoprotein B, the main protein constituent of LDL.
The positive study below of evolocumab, a monoclonal antibody that inhibits PCSK9 and lowers LDL cholesterol levels by approximately 60%, enrolled over 27,000 people with established cardiovascular disease and a LDL cholesterol of 1.8 mmol/L or higher. Compared to placebo, evolocumab treatment significantly reduced the risk of cardiovascular death, myocardial infarction, stroke, hospitalisation for unstable angina, or coronary revascularisation by 15% (95% CI 8%-21%). Overall evolocumab therapy (140 mg every 2 weeks or 420 mg monthly by s.c. injection) was tolerated well.
This study is a triumph for targeted biology. Biological therapies clearly provide very good target specificity and are generally well tolerated. The question is whether or not this approach will be suitable for primary prevention and if it could be used without the need for a statin? Another question is whether or not antibody therapies will be surpassed by RNA interference, e.g. Inclisiran, that may only have to be given infrequently via subcutaneous injection.
The obvious question is will targeted biological therapies eventually move from secondary to primary prevention? Preventing cardiovascular events is an obvious strategy for optimising life-long brain health; by protecting brain and cognitive reserve you increase your resilience to developing age-related neurodegenerative disease. I see a brave new world in which a significant proportion of the population will be receiving pharmacological interventions to optimise the ageing process.
Sabatine et al. Evolocumab and Clinical Outcomes in Patients with Cardiovascular Disease. N Engl J Med. 2017 May 4;376(18):1713-1722.
BACKGROUND: Evolocumab is a monoclonal antibody that inhibits proprotein convertase subtilisin-kexin type 9 (PCSK9) and lowers low-density lipoprotein (LDL) cholesterol levels by approximately 60%. Whether it prevents cardiovascular events is uncertain.
RESULTS: At 48 weeks, the least-squares mean percentage reduction in LDL cholesterol levels with evolocumab, as compared with placebo, was 59%, from a median baseline value of 92 mg per deciliter (2.4 mmol per liter) to 30 mg per deciliter (0.78 mmol per liter) (P<0.001). Relative to placebo, evolocumab treatment significantly reduced the risk of the primary end point (1344 patients [9.8%] vs. 1563 patients [11.3%]; hazard ratio, 0.85; 95% confidence interval [CI], 0.79 to 0.92; P<0.001) and the key secondary end point (816 [5.9%] vs. 1013 [7.4%]; hazard ratio, 0.80; 95% CI, 0.73 to 0.88; P<0.001). The results were consistent across key subgroups, including the subgroup of patients in the lowest quartile for baseline LDL cholesterol levels (median, 74 mg per deciliter [1.9 mmol per liter]). There was no significant difference between the study groups with regard to adverse events (including new-onset diabetes and neurocognitive events), with the exception of injection-site reactions, which were more common with evolocumab (2.1% vs. 1.6%).
CONCLUSIONS: In our trial, inhibition of PCSK9 with evolocumab on a background of statin therapy lowered LDL cholesterol levels to a median of 30 mg per deciliter (0.78 mmol per liter) and reduced the risk of cardiovascular events. These findings show that patients with atherosclerotic cardiovascular disease benefit from lowering of LDL cholesterol levels below current targets. (Funded by Amgen; FOURIER ClinicalTrials.gov number, NCT01764633 .).
BACKGROUND: Evolocumab is a monoclonal antibody that inhibits proprotein convertase subtilisin-kexin type 9 (PCSK9) and lowers low-density lipoprotein (LDL) cholesterol levels by approximately 60%. Whether it prevents cardiovascular events is uncertain.
METHODS: We conducted a randomized, double-blind, placebo-controlled trial involving 27,564 patients with atherosclerotic cardiovascular disease and LDL cholesterol levels of 70 mg per deciliter (1.8 mmol per liter) or higher who were receiving statin therapy. Patients were randomly assigned to receive evolocumab (either 140 mg every 2 weeks or 420 mg monthly) or matching placebo as subcutaneous injections. The primary efficacy end point was the composite of cardiovascular death, myocardial infarction, stroke, hospitalization for unstable angina, or coronary revascularization. The key secondary efficacy end point was the composite of cardiovascular death, myocardial infarction, or stroke. The median duration of follow-up was 2.2 years.
RESULTS: At 48 weeks, the least-squares mean percentage reduction in LDL cholesterol levels with evolocumab, as compared with placebo, was 59%, from a median baseline value of 92 mg per deciliter (2.4 mmol per liter) to 30 mg per deciliter (0.78 mmol per liter) (P<0.001). Relative to placebo, evolocumab treatment significantly reduced the risk of the primary end point (1344 patients [9.8%] vs. 1563 patients [11.3%]; hazard ratio, 0.85; 95% confidence interval [CI], 0.79 to 0.92; P<0.001) and the key secondary end point (816 [5.9%] vs. 1013 [7.4%]; hazard ratio, 0.80; 95% CI, 0.73 to 0.88; P<0.001). The results were consistent across key subgroups, including the subgroup of patients in the lowest quartile for baseline LDL cholesterol levels (median, 74 mg per deciliter [1.9 mmol per liter]). There was no significant difference between the study groups with regard to adverse events (including new-onset diabetes and neurocognitive events), with the exception of injection-site reactions, which were more common with evolocumab (2.1% vs. 1.6%).
CONCLUSIONS: In our trial, inhibition of PCSK9 with evolocumab on a background of statin therapy lowered LDL cholesterol levels to a median of 30 mg per deciliter (0.78 mmol per liter) and reduced the risk of cardiovascular events. These findings show that patients with atherosclerotic cardiovascular disease benefit from lowering of LDL cholesterol levels below current targets. (Funded by Amgen; FOURIER ClinicalTrials.gov number, NCT01764633 .).
Ray et al. Inclisiran in Patients at High Cardiovascular Risk with Elevated LDL Cholesterol. N Engl J Med. 2017 Apr 13;376(15):1430-1440.
Fitzgerald et al. A Highly Durable RNAi Therapeutic Inhibitor of PCSK9. N Engl J Med. 2017 Jan 5;376(1):41-51.
Comments
Post a Comment